Development of OTC medicines
Overall development and manufacture of Kampo products, crude medicine products,
vitamins, and other OTC medicines
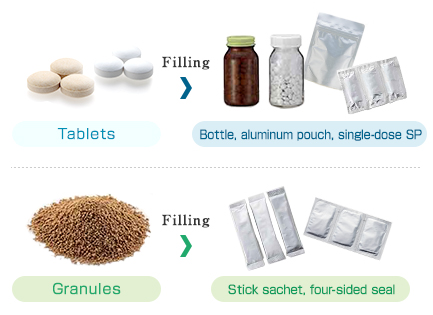
* Forms that can be processed: film tablets, sugar-coated tablets, wet granules, dry
granules, and others.

Flow of product development
 Client
Client
meeting- We confirm the needs for product development and summarize information on prescription design.
 Prescription
Prescription
design- We decide the ingredients of the medicine based on the result of the meeting.
 Preparation design
Preparation design- We discuss on preparing the medicine containing ingredients determined above (step 2)
 Manufacture of sample
Manufacture of sample
product- We manufacture the medicine designed above (step 3) in a lab‐scale as sample product, followed by the confirmation from client.
 Application
Application
for approval
and receipt of approval- We collect data, create documents for application for obtaining approval.
 Manufacturing
Manufacturing- We collect data and create documents for application for obtaining approval.
 Delivery of the product
Delivery of the product










